Yes. For many people with early to moderate androgenetic alopecia, Laser Phototherapy (LPT) is a safe, FDA-cleared, evidence-based option to stimulate hair density and slow hair thinning. But it’s not a miracle cure, and candidacy depends on your stage, scalp condition, and consistency.
Understanding How Laser Therapy Works
Laser Phototherapy, also called photobiomodulation, is a process in which red or near-infrared light penetrates the scalp and stimulates cellular activity — without heating or burning (unlike surgical lasers). The term laser stands for Light Amplification by Stimulated Emission of Radiation, but the “radiation” here is simply non-ionizing electromagnetic light, not the scary sci-fi kind.
The mechanism behind the magic
- The leading hypothesis: cytochrome c oxidase (CCO) in mitochondria absorbs red light, triggering more ATP production, improved redox state, and a release of nitric oxide (NO), which may improve microcirculation.
- This cascade can help dormant or miniaturized hair follicles reenter growth (anagen) and slow the shift to shedding (telogen).
- Other proposed effects include anti-inflammatory modulation, improved local blood flow, and antioxidant effects.
- That said — mechanism is biologically plausible but not completely settled in human follicle models.
Cold lasers vs surgical lasers — safety perspective
Cold lasers (Class 3R, ≤ 5 mW output) are designed to deliver non-thermal energy — i.e. they do not burn, cut, or damage tissue. The main risk is accidental direct eye exposure, which is why goggles or safety protocols matter. Because output is extremely low, typical consumer LPT devices are considered safe when used properly.
Am I a Good Candidate for LPT?
Hair loss type matters
LPT has the strongest evidence for androgenetic alopecia (AGA) (male and female pattern hair loss), also called pattern hair loss. Trials have primarily included participants with AGA, not scarring alopecia or alopecia areata.
If your hair loss is due to scarring, burns, lichen planopilaris, or long-standing bald patches with no follicles left, LPT’s benefits are less certain.
Stage of hair loss
Earlier is better. The presence of miniaturized follicles still capable of regrowth is key. Many RCTs enlist participants in early-to-moderate stages (e.g. Norwood II–V, Ludwig I–II). If your hair loss is very advanced (broad bald patches), results tend to be more modest.
Medical conditions & cautions
You should consult a professional if:
- You take photosensitizing medications (e.g. certain antibiotics, retinoids).
- You have active scalp lesions, open wounds, or a history of skin cancer on the scalp.
- You’re pregnant (risk data is limited).
- You have any conditions affecting skin sensitivity.
Always seek clearance from a dermatologist or trichologist before beginning.
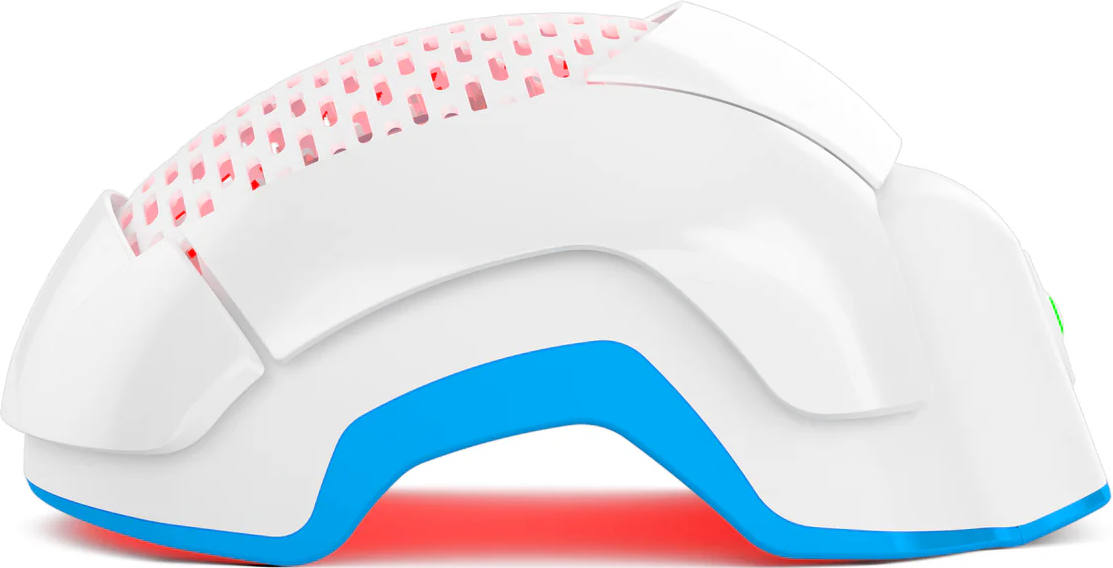
Theradome — First FDA-Cleared Wearable LPT Device
Clinical and regulatory backing
- The Theradome PRO LH80 was cleared in June 2013 under FDA 510(k) for women (K122950) and later for men; its 2018 510(k) update (K171775) covers both sexes and
- Norwood IIa to V in men, Ludwig I1–II2 in women. (FDA 510(k) summary, 2018)
Theradome’s EVO LH40 was cleared (K161046) as a lower-diode model variant. (FDA special 510(k) summary)
- According to the FDA 510(k) summary, the PRO helmet contains 80 coherent laser diodes, classed as Class 3R (≤ 5 mW per diode), designed for nonheating, hands-free 20-minute sessions.
- Clinical trials and clearances assert its intended use is to promote hair growth in AGA patients.
What sets Theradome apart
Theradome isn’t just another red-light gadget; it’s a clinically engineered, FDA-cleared system designed to deliver medical-grade precision in a wearable form.
Each Theradome helmet houses 100 % U.S.–made cold lasers, not LEDs, calibrated to emit a precise IntelliDose™ of 6.09 J/cm² across the entire scalp. That consistency matters: it means every follicle (front, crown, and temporal edges) receives an even, therapeutic level of photonic energy.
When those coherent red beams penetrate roughly 5 mm into the scalp, they reach the follicular bulge and dermal papilla, the biological “command centers” for hair cycling. Energy absorbed at this depth reactivates dormant follicles, extends the anagen (growth) phase, and can reduce shedding within about 30 days of consistent use.
The result is a full-coverage, hands-free treatment that quietly does its work while you read, scroll, or sip coffee. Built-in session timing, ergonomic balance, and soft internal padding make adherence almost effortless—because let’s be honest, the best regimen is the one you’ll actually stick with.
What to Expect from Laser Hair Growth Treatment
Timeline for results (realistic)
-
Weeks 0–12: You may notice reduced shedding, subtle thickening.
-
Weeks 12–26: Denser hairs, improved fullness.
-
6+ months: Visible volumetric change in many users — though results vary based on adherence and individual biology.
- Because LPT does not cure hair loss, continued use is necessary to maintain benefits.
Regimen, convenience & adherence
- Most clinical protocols: ~15–25 minutes/session, ~3× per week.
- Wearable helmets (like Theradome) allow multitasking (reading, watching TV).
- No downtime is required — treatments are noninvasive and easily integrated into routine.
Side effects & safety
- Commonly reported: slight tingling, mild redness, occasional temporary shedding.
- Serious adverse events are rare in controlled trials.
- Key safety measure: avoid direct eye exposure (class 3R hazard).
- Follow the device’s instructions carefully (e.g. protective eyewear where recommended).
What If I’m Not a Good Candidate?
- Minoxidil & finasteride remain cornerstone medical therapies — well validated with decades of RCTs.
-
PRP (platelet-rich plasma) offers promising results in more advanced cases, though protocols vary.
- Hair transplant surgery is a structural solution for extensive bald areas.
- In many cases, clinicians adopt multimodal plans (e.g. LPT + minoxidil) tailored to the person.
- Dysfunctional scalp health factors (nutrition, stress, hormonal imbalances) should be addressed in parallel.
Conclusion
Laser Phototherapy is not a magic bullet, but for many with early to moderate pattern hair loss, it offers a safe, evidence-backed, at-home tool to stimulate hair density. The science is solid, and the safety profile is favorable when guidelines are followed.
If your hair loss pattern aligns and you’re ready to commit (patience + consistency), a clinically designed device like Theradome may be a smart choice. Discuss with a trichologist or dermatologist, measure your baseline, and track progress with photos.
Interested? You can explore Theradome’s FDA-cleared helmets, and schedule a scalp consultation via our site.